About the study:
Epidemiological overview:
India is the second largest consumer of tobacco products in the world: 29% of the adult population uses smoked, smokeless, or both forms of tobacco. In the second-largest state of India, Madhya Pradesh (MP), about 34% of all adults (50% males, 17% females) and 21% of school personnel (28% males, 8% females) smoke and/or use smokeless tobacco. There is a dearth of rigorous systematic research testing the use of smartphones as a training-of-trainer approach in health behavior change or tobacco control. Although work has been done to test individual-level interventions using mobile devices (mHealth), research on using smartphones to train program implementers in Low and Middle-Income Countries (LMICs) is still in its infancy.
Context and problem statement:
Our study represents a major departure from the status quo of costly and time-intensive in-person training by testing a low-cost smartphone-based training strategy to scale up the implementation of a tobacco control EBI in a low-resource setting. It will demonstrate the feasibility of smartphone-based training and its effect on EBI implementation in a school setting to improve tobacco control in LMICs. It will present a novel method for collecting and analyzing implementation data via smartphones.
Research Question:
Program In-charges’ implementation of the TFT-TFS program, defined as implementing a minimum standard of core program components within the school, will be as good or superior in the smartphone training arm compared to the in-person training arm.
Study Aims and Objectives:
AIM 1.
Tailor the TFT-TFS program to the MP context and develop a smartphone-based training model centered on the systematic assessment of contextual factors in MP using the Consolidated Framework for Implementation Research (CFIR) framework
Approach:
Step 1: We conducted a three-part formative research study: (1) Key Informant Interviews (KIIs) with Department
of Education (DoE) leadership, principals, and Head Masters (HMs); (2) Focus Group Discussions (FGDs) with teachers;
and (3) School Observation visits (SOVs).
Step 2: Based on the Technical Working Group consensus, Creative Brief, and iterative feedback from participants,
we will tailor the existing in-person TFT-TFS training to ensure content relevance and usability in the MP context.
Methodology:
Step 1: Trained facilitators conducted KIIs and FGDs in Hindi and audio recorded them, with questions guided
by four CFIR domains: Intervention characteristics (TFT-TFS program), Inner Setting (schools), Outer Setting
(blocks, district), and Individual Characteristics (school teachers).
Step 2: Development of a smartphone-based training model to improve the quality and completion of program
implementation and tracking, using ‘gamification’ to improve user engagement. Standard use of this tracking
method across the two study arms allowed comparisons between the training models. We conducted formative
research in five districts of MP.
Outcomes:
Based on the iterative feedback from the participants, we tailored the existing TFT-TFS program to the MP context for both training models and developed the TFT-TFS Android-based smartphone app for training, implementation, and monitoring.
AIM 2.
Compare the fidelity, effectiveness, and cost of TFT-TFS program implementation for the two training models
Approach:
We conducted a two-arm, cluster randomized control trial, with schools as the unit of randomization, comparing (1) an in-person training model to (2) a smartphone-based training model. We randomly assigned High Schools (HS) and High-Secondary Schools (HSS), which met the eligibility criteria of having eight or more teachers, to one of two conditions.
Methodology:
We conducted a baseline survey in HS and HSS of two selected districts at three data points:
i. School Personnel Surveys – to assess tobacco use by type of tobacco; cessation; perceptions and
enforcement of school tobacco control policies; and awareness of and participation in the TFT-TFS program.
ii. Principals/HMs interviews – to assess tobacco policy implementation and tobacco control efforts
iii. School Observation Visits – to record indicators of tobacco policy implementation
We conducted an End line survey in the same schools at the same data points. Being the school’s administrative
authority, interviews of the Principals/HMs were conducted during baseline. However, post-implementation of
the TFT-TFS program, interviews of the Program In-charge were conducted i as they were the program implementers.
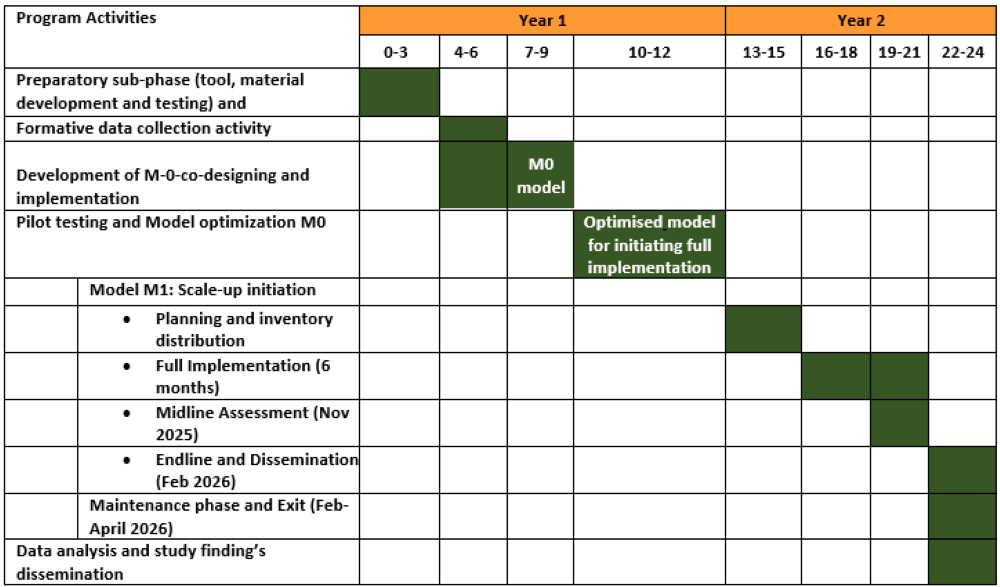
Timeline:
July 2021-June 2025
Program In-charges’ training:
In-person arm, we conducted three bi-monthly face-to-face training for six themes, using the paper-based
implementation manual. The training curriculum included demonstrations, group discussions, and opportunities
for behavioral rehearsal and feedback through role-plays.
In the Smartphone arm, we conducted only one face-to-face orientation of the TFT-TFS Android app during
September-October 2023. Further, to implement the six themes, the program in charge trained themselves
using the app.
Expected Outcomes:
We expect to have demonstrated the comparative effectiveness of each training model in implementing the TFT-TFS program and identified any differences in implementation fidelity, cost, and reach.
AIM 3.
Identify factors affecting program implementation after in-person vs. smartphone-based training using a mixed-methods design
Approach:
Based on our prior research experiences, we selected factors within four domains of CFIR as in Aim 1. Analysis of these factors allowed us to (1) examine the relation between quantitative CFIR constructs and implementation data from Aim 2, and (2) explore how these factors influence TFT-TFS implementation, through qualitative interviews with Program In-charges/HMs/principals.
Methodology:
We used an explanatory sequential mixed methods evaluation design, using quantitative survey data
from Aim 2 (End-Line survey) and post-implementation qualitative data among the
Program In-charges/HMs/Principals/teachers in each arm.
Mixing quantitative and qualitative data: We will present side-by-side comparisons of findings in each
arm. Table cells will contain the quantitative/qualitative results for each CFIR construct and the mean
number of components implemented, cessation, cost, and reach. With our Technical Working Group, we will
synthesize participants’ solutions to challenges and suggestions for future improvements.
Expected Outcomes:
We expect to have identified contextual factors that explain variations in TFT-TFS implementation for each training model.
Type of Study:
Behavioral Mixed-method study using Comparative-effectiveness trial
Study Participants:
Teachers from Government HS and HSS of the state education system of MP
Collaborators:
Dana-Farber Cancer Institute (DFCI) and Harvard T.H. Chan School of Public Health (HSPH) in Boston, USA
Principal Investigators:
Dr. Mangesh S. Pednekar and Dr. Eve M. Nagler
Co-Investigators:
Dr. Prakash C. Gupta and Dr. Glorian Sorensen
Current Status:(July 2021 – December 2024)
AIM 1
We have published an article titled “Smartphones: A Catalyst for Tobacco Control Training in India” in the Proceedings of the 23rd European Conference on e-Learning 2024 on the TFT-TFS smartphone development process
https://papers.academic-conferences.org/index.php/ecel/article/view/3065
AIM 2
We are working on manuscript publications of the baseline data. In addition, we are working on the manuscript publications of cost-and-time data for the training of the Program in-charges, TFT-TFS program implementation, and reach.
AIM 3
We are working on the translations of the KIIs and FGDs conducted as part of the post-implementation qualitative research, and the data cleaning of the end-line surveys.
About the study:
Epidemiological overview:
India faces one of the highest burdens of air pollution-related health risks globally. Exposure to airborne pollutants like polycyclic aromatic hydrocarbons (PAHs), aldehydes, and heavy metals has been linked to cancers of the aerodigestive tract, particularly lung cancer (LC) and head and neck cancers (HNC). The relatively low prevalence of smoking in India presents a unique opportunity to study these associations without the major confounding effects of tobacco smoke.
Context and problem statement:
While numerous studies globally have identified the harmful effects of air pollution, there is limited biomarker-based data from non-smoking populations. India offers a unique environment due to its high ambient air pollution and prevalent smokeless tobacco (SLT) use. However, research infrastructure and biomarker analysis capacity remain limited. This study addresses these gaps through a US-India partnership, aiming to understand exposure-disease associations and build long-term biomonitoring capacity at the Centre for Cancer Epidemiology (CCE), Mumbai.
Study Objective:
To determine the association between air pollution-related biomarkers and cancer risk in Indian non-smokers using established and novel biomarker assays, while simultaneously building local laboratory capacity for future biomonitoring research.
Specific Aims:
• Aim 1:
Assess the association of air pollution-related biomarkers with lung cancer (LC) in Indian non-smokers using stored biospecimens from an established LC cohort.
• Aim 2:
Determine the contribution of air pollution exposure to risk of SLT-associated HNC in Indian non-smokers.
• Aim 3:
Examine biomarker phenotypes in healthy non-smokers across varying levels of air pollution exposure using personal air sampling devices and biological matrices (blood, urine, oral cells).
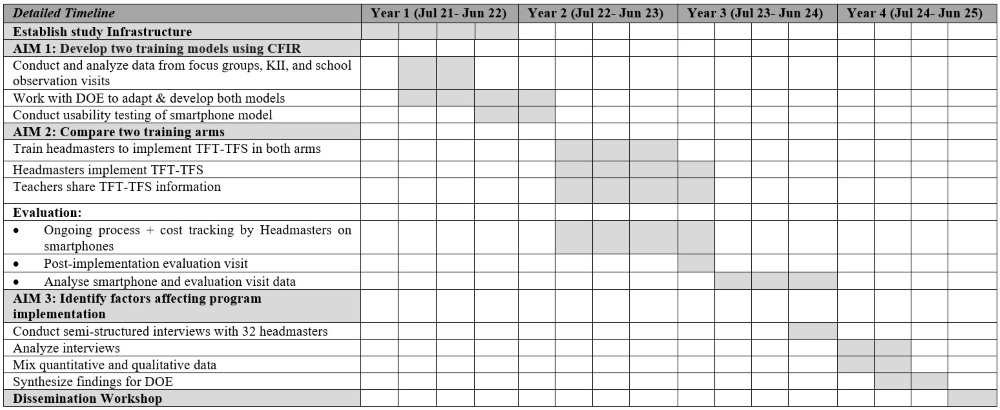
Methodology:
>• Case-control studies (Aims 1 & 2):
Utilize plasma and buffy coat samples from existing LC and HNC cohorts at CCE. Biomarkers include PAH-albumin adducts, aldehyde-adducts, heavy metals, tobacco-specific nitrosamines, and oxidative stress indicators.
• Lab capacity building:
A bioanalytical laboratory will be established at CCE with assay development and training support from the University of Minnesota.
• Cross-sectional study (Aim 3):
This aim will carried out by Healis in collaboration with CCE, ACTREC.
Overall approach AIM 3:
o Recruitment
Around 150 individuals will be recruited; 50 per exposure group, in the study based on the eligibility criteria.
Exposure groups will include:
(i) Street vendors working at a stall in open air, in proximity to traffic, 5 days a week, at least 8 hours a day;
(ii) Restaurant workers spend most of the work-time and have cooking as a major responsibility
(e.g., cooking 5 days a week,at least twice daily);
(iii) Office workers work in enclosed environments not in direct proximity to heavy traffic (at least 2 km away)
at least 5 days a week, 8 hours a day, and not using wood- or coal-burning stoves.
o Study components for data collection
Filling questionnaire: We will conduct the consent and questionnaire procedures.
Biological samples: prior to collecting biological samples, only for participants who agree to this procedure
Air samplers: Study staff will wear the device, being in close proximity to the selected/consented participant.
Personal samplers to be worn for 8 hours, 3 consecutive days. Air sampling instrument (Andersen impactor) to be used
for 1 day only.
Timeline: 2021-2026
Research Question:
Is the uptake of air pollution-related chemical carcinogens associated with an increased risk of lung and head and neck
cancers in Indian non-smokers?
This question is examined using a panel of established and novel biomarkers to explore the
biological link between environmental exposures and cancer risk.
Lung Cancer Risk (Aim 1):
Do Indian non-smokers with lung cancer have higher levels of air pollution-related biomarkers (e.g., PAH-albumin adducts, aldehydes, heavy metals, oxidative DNA damage) compared to matched cancer-free controls?
Head and Neck Cancer in SLT Users (Aim 2):
Among non-smoking SLT users, does higher exposure to air pollution—as reflected by biomarker levels—contribute to increased risk of oropharynx and larynx cancers?
Biomarker Profiles and Pollution Exposure (Aim 3):
Do healthy non-smokers living in areas with different levels of air pollution exhibit distinct biomarker profiles related to pollutant exposure?
Expected Outcomes:
• Provide evidence linking air pollution biomarker levels with cancer risk in non-smokers.
• Enable development of preventive policies targeting environmental carcinogens.
• Establish infrastructure for future biomarker-based cancer epidemiology research in India.
Type of Study:
Environmental and cancer epidemiology; mixed-methods biomarker-based study
Study Participants: Non-smoking individuals, including:
• LC and HNC patients from existing CCE cohorts
• SLT users with and without HNC
• Healthy non-smoking adults with varied environmental exposure
Collaborators:
• University of Minnesota, USA
• Centre for Cancer Epidemiology (CCE), Mumbai, India
• Healis Sekhsaria Institute for Public Health
Principal Investigators:
• Prof. Irina Stepanov (Regents of the University of Minnesota)
• Dr. Rajesh Dixit (Centre for Cancer Epidemiology, Tata Memorial Centre, ACTREC)
Co-Investigators:
• Dr. Prakash C, Gupta (Healis Sekhsaria Institute for Public Health)
• Prof. Dorothy K. Hatsukami (Regents of the University of Minnesota)
• Prof. Christopher J. Hogan(Regents of the University of Minnesota)
• Prof. Samir S. Khariwala (Regents of the University of Minnesota)
• Prof. Xianghua Luo (Regents of the University of Minnesota)
• Dr. Peter Villalta (Regents of the University of Minnesota)
• Dr. Sharayu Mhatre (Centre for Cancer Epidemiology, Tata Memorial Centre, ACTREC)
Status (as of April 2025):
1. Bioanalytical lab has been establishment at CCE
2. Sample selection and protocol finalization for Aim 1 and 2 is completed
3. Data collection for the Aim 3 is started
About the Study:
Epidemiological overview:
Dental caries, is a prevalent non-communicable disease, not only among the adult population but also a chronic ailment among children. In India, according to the National Oral Health Survey, dental caries prevalence in India was 51.9%, 53.8%, and 63.1% at 5yrs, 12yrs and 15yrs respectively indicating a significant public health challenge among growing children. The geographic distribution of dental caries depicts higher prevalence in western India (72%) followed by northern (57%), central (56%), southern (51%), and eastern (36%) parts of India.
Context and Problem Statement:
Maharashtra has reported dental caries in 79-84% of children, highlighting the need for effective preventive interventions among children. Effective preventive measures for dental caries involve adopting oral health-promoting behaviours, such as regular tooth brushing, reducing sugar intake, and attending routine dental check-ups. A conducive school environment wherein oral health interventions are delivered has been effective in improving oral health behaviours and in the prevention of dental caries among children. Therefore, developing a sustainable implementation model would be pivotal in promoting oral health behaviours and for the prevention of oral diseases among young children.
Aim:
The study aims to develop and implement a school-based tooth brushing and oral health education (OHE) scalable delivery model with high coverage for improving oral health amongst primary school children in Sindhudurg district Maharashtra.
Specific Study Objectives: Primary Objectives:
Objective 1:
To assess current tooth brushing practices & identify barriers and facilitators to implement an oral health intervention delivery model among primary school children, parents, teachers and other key stakeholders
Approach:
The formative phase aims to comprehensively understand and identify key aspects, including stakeholders, existing programs, available resources, and the current state of awareness and practices related to oral health in schools and communities. Our exploration delves into understanding the contextual barriers and facilitators among various stakeholders, including beneficiaries (children and parents), and service providers (health and education staff) to implement the model. This detailed examination is vital for designing a contextualized delivery model that is not only scalable and feasible but also acceptable, equitable, and widely acceptable.
Research Question:
What are the barriers and facilitators to implementing an oral health intervention delivery model among primary school children, parents, teachers and other key stakeholders?
Methodology:
We used a blend of qualitative methods for data collection, including direct observation, in-depth interviews, focus group discussions, and non-formal interactions, using semi-structured guides for our detailed situational analysis. Further, secondary data regarding health-related programmes in the district and school textbook evaluation for oral health information have been collected from the health/education system and school records. The analysis of formative data was guided by the Consolidated Framework for Implementation Research (CFIR) framework, categorizing implementation predictors into five domains.
Expected Outcomes:
Formative data collection and analysis have been completed leading to a comprehensive identification and understanding of the barriers and facilitators influencing the implementation of the oral health intervention delivery model.
Objective 2:
To co-develop and implement a context-specific oral health intervention delivery model and to support its iterative optimization.
Approach:
Co-development and Co-designing approaches were used to develop a context-specific implementation delivery model. Our model were align with the Theory of Change, facilitating the necessary behaviour change
Research Question:
How can a context-specific oral health intervention delivery model be co-developed, implemented, and iteratively optimized to improve oral health outcomes?
Methodology:
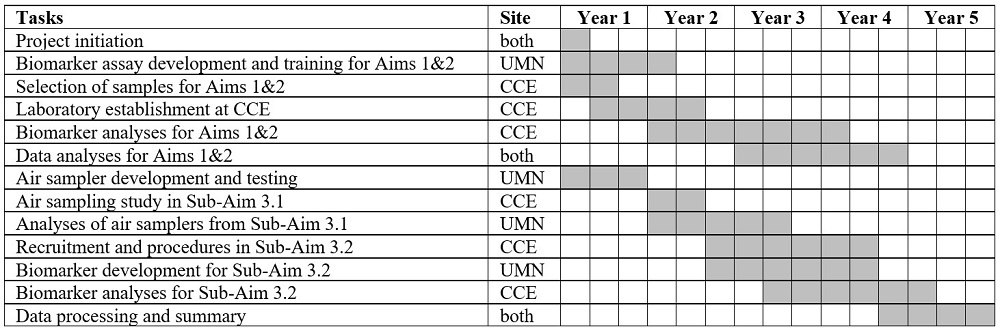
We used the insights and findings of the formative data analysis to develop the implementation delivery model (M-0). The core implementation strategy developed is to enhance the capacity of implementers (Community Health Officer (CHO), and School teachers), by utilizing the Train the Trainer Model (ToT). The CHOs in the selected study blocks were trained by a dental surgeon from the health system. The CHOs further trained a Master Trainer (designated school teacher) from the schools, who further trained all the other teachers in their respective schools. All trained teachers had implement tooth brushing and oral health education activities at their schools. The implementation model (M-0) had undergone pilot testing in 20 schools, ensuring diversity across various strata (rural/urban and government/private schools) in the study site. We had continuously track process indicators for the outcomes' acceptability, adoption, fidelity, and coverage and iterate the model until it is optimized to a maximum extent in the pilot period. Qualitative/non-formal interactions were done at different stakeholder levels to understand barriers and facilitators for implementation in the pilot testing period and to understand the acceptability of the model. Process tracking forms/activity checklists were used by the implementers (School teachers) to record all the intended activities for the pilot testing of the model. The pilot findings were discussed in a Co-Development Workshop with the relevant stakeholders and a refined Model M-1 was collaboratively created. The co-development process had involved brainstorming on the identified potential barriers/facilitators, optimization of pilot strategies, finalizing the potential implementation strategies, planning the delivery mechanisms, and contextualizing the model to the study site. With the co-development process and by reaching at least 80% optimization in the pilot period, the model M-1 is ready to be implemented in selected schools (up to 400) in the district during the implementation period.
Timeline: March 2024- April 2026
Expected Outcomes:
Based on the pilot testing findings of the implementation delivery model (M-0) a refined Model M-1 is being developed for implementation.
Objective 3:
To evaluate the optimized model for acceptability, adoption, fidelity, coverage, and cost.
Approach:
In the pilot and implementation period, we had comprehensively evaluated and understand the acceptability and feasibility of implementing the model, along with any barriers to the implementation strategies. Further, fidelity and coverage measures of the model implementation had allowed us to refine and optimize the implementation delivery model based on real-world testing and feedback. The acceptability of the programme will be measured as 80% of the relevant stakeholders perceive that the tooth brushing and oral health education intervention is agreeable and satisfactory. The adoption was measured by at least 80% of schools agreeing to participate in the program and having at least 80% teacher and CHO participation rate in the training. The coverage was measured as at least 80% of the schools implementing the tooth brushing sessions for at least 3-5 days/week and have completed at least 3 out of 4 OHE sessions. In addition, at least 80% of the children present on the session day had participated in the sessions. The fidelity was measured as at least 80% of the schools implementing the programme as intended. The costs will be measured as implementation costs for implementing the program activities.
Research Question:
What is the impact of the optimized model on acceptability, adoption, fidelity, coverage, and cost?
Methodology:
Process tracking forms/activity checklists will be used by the implementers to record all the intended activities during the implementation. Qualitative/non-formal interactions will be done at different stakeholder levels to understand the acceptability of implementing the model. Further, an observational checklist will be used to record the fidelity measures by the research team during the implementation period (sampled schools).
Expected Outcomes:
The implementation delivery model (M-1) will be implemented and the implementation outcomes acceptability, adoption, fidelity, coverage, and cost will be assessed.
Secondary Objectives:
Objective 4:
To assess the outcome of the optimized model to improve oral hygiene behaviours and its impact on dental caries.
Approach:
We will also measure the impact outcomes such as a change in oral health behaviours among beneficiaries (Healthcare workers, and teachers) and mean DMFT/deft and Oral Hygiene Index scores among children at baseline and end line.
Research Question:
How effective is the optimized model in improving oral hygiene behaviors and reducing the incidence of dental caries?
Methodology:
The oral health behaviours will be recorded using KAP (knowledge, attitude, and practices) questionnaires from a random sample of healthcare workers, and teachers. The oral hygiene status (oral hygiene simplified-debris index) and dental caries status (dental caries prevalence and DMFT/deft index) among children will be recorded using the clinical examination performa. All these will be recorded as cross-sectional data at the baseline and end-line of the study.
Expected Outcomes:
The effectiveness of the optimized model will be assessed based on changes in oral health behaviors, oral hygiene status, and dental caries status from baseline to endline.
Acknowledgment:
This project initially received partial funding under the National Health Research Priority Projects by the Indian Council of Medical Research (ICMR), New Delhi.
Principal Investigators:
Dr. Mangesh. S. Pednekar and Dr. Prakash. C. Gupta
Co-investigators:
Mr. Sameer Narake, Dr Namrata Puntambekar
Current Status:
Implementation of the Oral Health delivery Model is ongoing.