About the study:
Epidemiological overview:
India is the second most populous country in the world, and the third largest producer and consumer of tobacco. The country has a long history of tobacco use and a variety of ways of smokeless tobacco use and smoking, of which cigarettes form only a minor part. Almost all forms of tobacco use carry serious health consequences. However, if we estimate the death and disease burdens from tobacco use in India only based on cigarette smoking, we may grossly underestimate the results. Previous estimates of tobacco-attributable mortality in India were based on the results of cohort studies in rural areas of Ernakulam District, Kerala, and in Srikakulam District, Andhra Pradesh. These studies followed cohorts of over 10,000 villagers aged >=15 years in a house-to-house approach for 10 years. Thus, we obtained accurate estimates of all-cause mortality, which allowed us to make estimates of the relative risks for different tobacco use. By using conservative figures and employing 1986 mortality data for the whole of India, researchers estimated that tobacco-attributable mortality in the country amounted to 630,000 deaths per year. Since there was no data on the causes of death, we did not calculate cause-specific mortalities. To get cause-specific tobacco-attributable mortality in India, we started cohort study in Mumbai in 1990.
Context and problem statement:
To study tobacco use and mortality among adult aged 35 >= years.
Study objective:
To assess cause specific mortality attributable to the use of tobacco in the form of smoking and chewing.
Research Question:
Will tobacco users have a greater death rate than non-users?
Aim:
To recruit adults >=35 years old and do baseline survey & subsequent follow up survey.
Approach:
The sampling frame used was the electoral rolls, which provided the name, age, sex, and address of all individuals aged >18 years. The rolls were fairly complete since almost everyone aged >18 years is entitled to vote and they are updated before every major election through house-to-house visits. After selecting a polling station, all individuals aged >=35 years on the appropriate electoral list were approached house-to-house by investigators for an interview.
Methodology:
During 1991-97, we recruited 148,173 men and women aged >=35 years in Mumbai city, using the voters’ list as the selection frame. We conducted the first follow-up during 1997-2003 and the second follow-up during 2004-2018.
Outcomes:
The study reported for the first time about the excess all-cause and cause-specific mortality from various forms of tobacco use other than cigarette smoking. The study concluded for the first time that bidi smoking is no less hazardous than cigarette smoking, and smokeless tobacco use may also result in significantly increased mortality.
Type of Study:
Prospective Cohort Study
Study Participants:
Permanent residents of the city of Mumbai aged 35 years and older.
Timeline:
Started 1990 - second follow up ended - 2018
Collaborators:
None
Principal Investigators:
Dr. Prakash C. Gupta
Dr. Mangesh S. Pednekar
Current Status: (Dec 2024)
1. Data analysis is going on.
Publications:
MCS solely
Asia cohort consortium
NCD risk factor collaboration
Global burden of disease group
Global Cardiovascular Risk Consortium
About the study:
This study was done to perform a case-control analysis to test the hypothesis that candidate SNPs are associated with increased BcCA risk and that subjects with both poor (pro-inflammatory, high-fat) diets and candidate risk genotypes have even greater BrCA risks compared to subjects without a risk allele and with more healthy diets.
Type of study:
Genetic and Dietary Case-Control study of Breast Cancer
Timeline:
2009-2013
Collaborators:
Arnold School of public Health, Tata Memorial Hospital and Healis – Sekhsaria Institute for Public Health.
Principal Investigator:
Dr. Jim Burch, James Herbert (Co-investigator), Susan E. Steck (Co-investigator), Dr. Rajiv Sarin (Co-investigator), Dr. P.C. Gupta (Co-investigator)
Study Participants:
Altogether, we had 2357 diet forms (FFQs) received, coded and shared from ACTREC so far with the team. Details of the Food frequency questionnaires are as follows:
BGSC (Sporadic Cases) -1183
BGFC (Familial Cases) -064
BGSN (Sporadic Normal) -1086
BGFN (Familial Normal) -024
Publications:
- Case control study of breast cancer in India: role of period3 clock gene length polymorphism and chorotype.
- Stage at diagnosis and relative differences in breast and prostate cancer incidence in India: comparison with the United States.
- Decreased survival with mastectomy vis-à-vis breast conserving surgery in stage II and stage III breast cancers: a comparative effectiveness study.



About the study:
The broad objective of the TCP India Project is to evaluate and understand the prevalence and patterns of tobacco use and impact of tobacco control policies of the Framework Convention on Tobacco Control (FCTC) as they are implemented in low- and middle-income countries (LMICs) participating in the International Tobacco Control Policy Evaluation Project (the ITC Project).
Note: The name Tobacco Control Project (TCP) is used in reference to the India Project instead of International Tobacco Control (ITC) Project, used with other countries, because in India the abbreviation ITC also refers to the Indian Tobacco Company.
Type of study:
Longitudinal cohort study
Timeline:
01 Feb, 2017 to 31 Dec, 2021
Collaborators:
University of Waterloo, Canada
Study Participants:
A total of 2000 tobacco users and 600 non-users from urban and rural population sample were selected from each of the four states of Maharashtra, Madhya Pradesh, West Bengal and Bihar for the study. Wave 1 and Wave 2 of the TCP India project have already been executed. As a follow-up, in Wave 3 re-contact of participants recruited in previous waves across these four states have been done.
Principal Investigators:
Dr. Mangesh S Pednekar, Dr. Geoffrey T. Fong
Co-Investigator-
Dr. Prakash C. Gupta
Publications:
Current Status:
As of September 2022
• Press conference on “Tobacco Control Policy Evaluation Project” was conducted on May 30, 2022.




About the study:
This study aims to prospectively measure Community Tobacco Environmental (CTE) factors (i.e., objective assessments of community level compliance with tobacco control laws, availability of all forms of tobacco products including gutkha and e-cigarettes, and the presence of tobacco vendors and advertisements). The study will also estimate whether CTE factors are longitudinally associated with adolescent tobacco use initiation and trajectories
Type of study:
Longitudinal Cohort study extending over a period of five years
Timeline:
August 2016-December 2021
Collaborators:
1)University of Michigan, USA
2)University of California (UCLA), Los Angeles, Fielding School of Public Health, USA
Principal Investigators:
Dr. Mangesh S Pednekar, Dr. Ritesh Mistry.
Co-Investigators:
Dr. Prakash C. Gupta, Dr. William McCarthy, Prof. Trivellore Raghunathan,
Dr. Namrata Puntambekar.
Study Participants:
Approximately 2000 adolescents and their parents will be surveyed and followed up over a period of four years in two cities in India- Mumbai and Kolkata.
Publications:
Longitudinal study of adolescent tobacco use and tobacco control policies in India.
Family functioning within the context of families with adolescent children in urban India.
Current Status:
As of September 2022
• Wave 3 data collection is completed and currently working on developing survey tools for Wave 4.
• All the tools (consent, scripts, and questionnaire) for Wave 4 have been finalized in all the languages (English, Hindi, Marathi, and Bengali).
• Community mapping has been completed in all the areas in Mumbai and Kolkata.




About the study:
Epidemiological overview:
According to World Health Organization (WHO), by 2030, more than 8 million people globally are expected to die from tobacco-related causes, 80% of whom will be from LMICs. In India in 2010 alone, an estimated 930,000 people died from smoking, and in 2008, an additional 368,000 deaths were attributed to smokeless tobacco use, illustrating the complex effects of the use of multiple forms of tobacco. Reflecting these trends, India has one of the highest oral cancer rates in the world. In 2010, the prevalence of tobacco use among men in India was 48% and among women was 20%.
Context and Problem Statement:
The National Cancer Institute (USA) formed its Center for Global Health aimed at reducing the global burden of cancer. Although India was an early signatory to the Framework Convention on Tobacco Control, few resources were available to support tobacco use cessation, the prevalence of quitting was low and, few social norms supported quitting. Ensuring the availability of adequate evidence and making use of available evidence to respond by pressing public health issues, particularly in light of the lag time between efficacy/effectiveness research and implementation of evidence-based intervention in practice.
Study objective:
To identify effective strategies for broad-based implementation of the evidence-based tobacco control intervention (tested in schools of Bihar) that could be embedded in existing organizational infrastructures and could accommodate the realities of low-resource settings. The study was implemented in schools in Bihar.
Research Question:
To achieve this objective our central research question was: will this evidence-based intervention be successfully adopted, implemented, and maintained through existing channels using the proposed implementation model?
Accordingly, the Specific aims of the study are:
Aim 1.
Determine the extent to which this implementation model meets acceptable rates of program adoption, implementation, and reach of the program among schools in Bihar.
Approach:
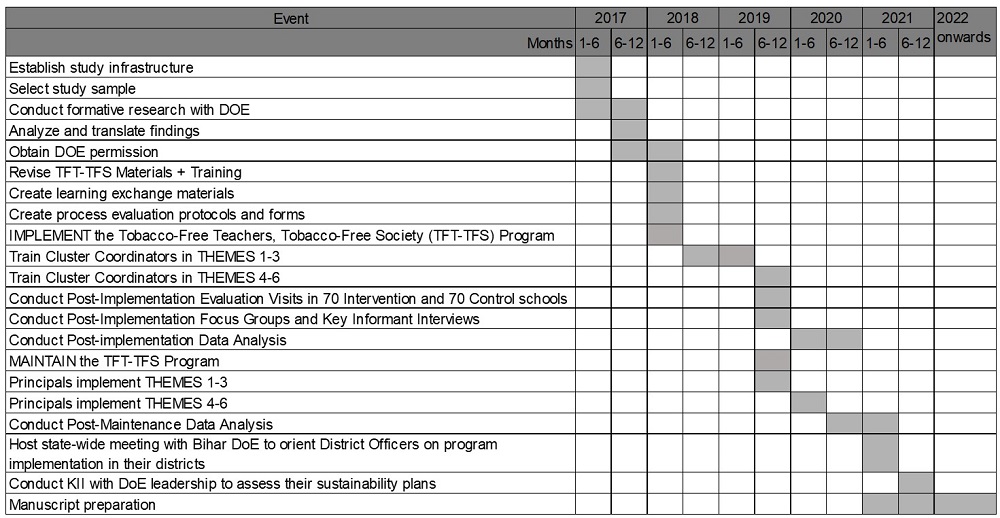
In India, the education system is structured hierarchically, with schools nested within clusters, clusters within blocks, and blocks within districts. The dissemination of new curricula typically follows a cascade model, progressing through this nested structure. For this study, we leveraged this existing framework by training cluster coordinators—those responsible for curriculum training—to build the capacity of school principals to implement and maintain the TFT-TFS program effectively using the Trainings-of-Trainers strategy. Cluster coordinators were trained to provide ongoing training, guidance, and support to school principals throughout the implementation phase.
Methodology:
A randomized control trial (RCT) design was employed. After securing the support letter from three districts, selected two blocks from each of the three districts and randomly assigned one block per district to the intervention and control arm. A total of 219 school headmasters from 46 clusters within three blocks of the intervention arm were trained by 46 cluster coordinators at their respective cluster resource centers. Trained headmasters or designees implemented the TFT-TFS program in their schools.
Outcome:
The implementation model was adopted and implemented in the schools of Bihar and Headmasters were trained.
Aim 2.
Determine program effectiveness in increasing the implementation of tobacco control policies and in promoting tobacco use cessation in schools.
Approach:
To assess program effectiveness in improving tobacco policy implementation, increasing tobacco use cessation, and monitoring secular trends in tobacco control, we conducted two evaluation visits: one before and one after program implementation. These visits were complemented by surveys administered to headmasters and school personnel, as well as the use of standardized observation checklists within the schools.
Methodology:
For assessing the effectiveness of the intervention activities, 70 schools were randomly selected from a total of 219 schools in the intervention block, and 70 schools were randomly selected from 224 schools in the control blocks. This gave us a sample size of 429 teachers from intervention schools and 331 teachers from control schools. Surveys and observation checklists were standardized, using validated instruments designed to measure both the implementation of tobacco control policies and tobacco use cessation efforts. Baseline data was collected before the intervention to compare pre-and post-implementation outcomes.
Outcome:
The program was effective in increasing the implementation of tobacco control policies.
Study Timeline: January 2017- 31st December 2023
Aim 3.
Determine the feasibility of building the capacity of cluster coordinators to train and support principals in program implementation and maintenance in schools, and for the DoE to sustain the program.
Approach:
After analyzing post-maintenance data, a state-wide meeting was hosted with the Department of Education (DoE) to orient district officers in Bihar on implementing the program in their districts. Key informant interviews were conducted with DoE leadership to assess sustainability plans.
Methodology:
Over years 2-4, regular consultation meetings were held with DoE to review the implementation and maintenance of the TFT-TFS program in schools. Findings were used to refine the implementation model and develop a sustainability plan, which included: • Leadership support by aligning the program with DoE priorities. • Integration of training and technical assistance roles into DoE job descriptions and performance metrics. • Resource allocation, budgeting, and cost-effective production of program materials. • Monitoring and evaluation by using DoE's existing tracking systems to evaluate the program over time. Collaboration with DoE to ensure maintenance strategies and embed responsibilities into existing DoE roles.
Outcome:
A self-help guide for implementing the TFT-TFS program was developed and disseminated to 25,000 schools in Bihar. An assessment of dissemination in 12 blocks from 6 districts showed that 96% of headmasters received soft copies of the guide
Type of Study:
Intervention dissemination.
Study Participants:
Headmasters and school teachers.
Collaborators:
Dana-Farber Cancer Institute & Harvard School of Public Health, USA
Centre for Health Decision Science, Harvard School of Public Health, USA.
Principal Investigators:
Dr. Prakash C. Gupta, Dr. Glorian Sorensen
Co-Investigators:
Dr. Mangesh S. Pednekar, Dr. Eve M. Nagler, Dr. K. Viswanath, Dr. Harry A. Lando, Dr. Jane Kim, Dr. Dhirendra N. Sinha
Publications:
Dissemination:
The dissemination of a self-help guide for implementing the TFT-TFS intervention in Bihar and Maharashtra was published and released in regional languages during a press conference.
Current Status: July 2024- December 2024
Completed project: Data analysis and manuscript under preparation.
About the study:
This study investigates the relationship between carcinogen content in smokeless tobacco (SLT) products and relevant exposures as well as oral/head and neck cancer (OHNC) risk in users of these products, while concurrently building capacity for a sustainable tobacco carcinogenesis research program in India.
Type of study:
Laboratory Epidemiology
Timeline:
August 2017- July 2022
Collaborators:
Department of Otolaryngology, University of Minnesota, US
Masonic Cancer Center, US
Tata Memorial Hospital, India
Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Navi Mumbai, India
Principal Investigators:
Dr. Samir S. Khariwala, Dr. Irina Stepanov, Dr. Pankaj Chaturvedi
Co- Investigators:
Dr. Prakash C. Gupta, Dr. Vikram Gota, Dr. Dorothy Hatsukami, Dr. Saonli Basu
Publications:
Observed Circumvention of the Gutka Smokeless Tobacco Ban in Mumbai, India.
Study Participants:
Patients with oral head and neck cancer and healthy smokeless tobacco users.
Current Status:
As of September 2022
• The web portal has been designed and is currently under preparation -which will display all necessary project information on the Healis website.
• A smokeless tobacco review paper has been drafted and circulated with the full team for review.
• Continued storage of tobacco product samples obtained from recruited patients as well as smokeless products collected as a part of the repository.
• Paper under process titled “The urgent need for product regulation and public education to reign in the deadly toll of smokeless tobacco in India.”

About the study:
Epidemiological overview:
Asian Indians have high rates of diabetes, prediabetes and cardiometabolic risk factors; some countries, like India, are acutely affected. Lifestyle changes, including weight loss, increased physical activity, and improved diet quality, have been shown to prevent or delay diabetes and reduce cardiometabolic risk factors such as elevated glucose, plasma lipids, and blood pressure. Despite the evidence supporting the use of lifestyle interventions to prevent hypertension and diabetes and to improve glucose tolerance, their translation in real-world settings has been challenging. Worksite-based health interventions have proven to be effective in improving employee and worksite health. By providing resources and leveraging them at the workplace, these interventions overcome barriers to healthy lifestyle choices, as individuals spend a significant amount of time there. The environment in which an individual works has the potential to influence their health-related behaviors and promote behavioral change.
Context and problem statement:
Even though there is evidence supporting the effectiveness of lifestyle interventions at worksites, the success of these interventions depends on developing interventions grounded in an integrated framework that aligns with workplace governance. This is essential for institutionalization and achieving impact on scale.
Study Objective:
The main objective of our study is to assess the effectiveness of two interventions – a) canteen plus behavioral change and b) canteen only - in reducing cardiometabolic risk among employees at a worksite in India. We will evaluate the process outcomes.
Research Question:
To meet our objective, our central research question was: will the worksite multi-component intervention reduce cardiometabolic
risk among the pre-diabetic and/or pre-hypertensive employees of the worksite?
The specific aims of the study are:
AIM 1.
To facilitate the adoption and implementation of an existing evidence-based canteen intervention and behavior change intervention (Diabetes Prevention Program-DPP) to increase healthy eating habits in a worksite canteen environment.
Approach:
We facilitated the adaption and implementation of an existing Evidence Based Interventions’. DPP and Canteen Intervention to increase the healthy eating habits at the worksite environment. We explored the perceptions, provisions, drivers and barriers to healthy eating from worksite management, canteen operators through in-depth interviews (IDI) and from canteen users through focus group discussion (FGD). We administered ‘canteen intervention rating checklist’ to rank intervention components according to the ease of implementation by worksite management. Based on these findings, we contextualized and tailored the canteen intervention and DPP to suit the microenvironment needs of the worksite.
Methodology:
We assessed worksite canteens’ ability to provide healthy food through structured observations. We used a checklist to evaluate the food served, food options, portion sizes, point of choice, and information provided at the canteen. This helped us determine the potential for worksite canteen operators to increase the availability of healthy foods. Acquired the permission from the worksite management and the canteen operator; and observed the administration of the checklist.
Outcome:
The Canteen intervention and DPP was adapted, contextualized, tailored for the worksite environment and implementation plan was developed.
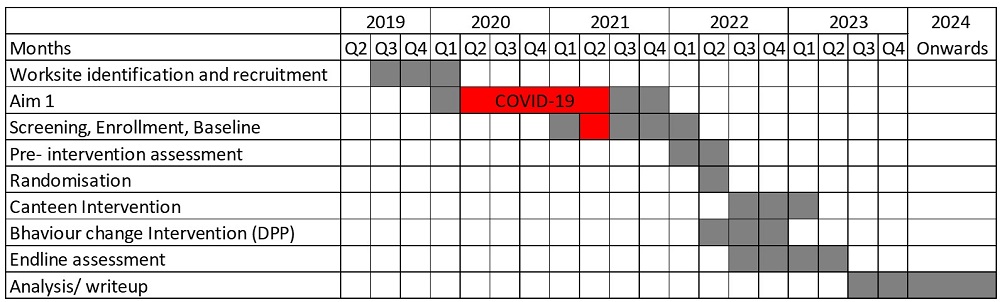
Timeline: June 2019- December 2023
AIM 2.
To measure the effectiveness of a multi-component worksite intervention to reduce Cardio-metabolic risk.
Approach:
Evaluating and comparing the changes assessed the effectiveness in the number of employees meeting two or more cardio-metabolic risk factors (ranging from 0 to 3). Success was defined by meeting specific criteria, including a 0.5% decrease in HbA1c, a 5 mm Hg reduction in systolic blood pressure, and a 10 mg/dl decrease in plasma triglycerides. These criteria were measured and compared at pre and post intervention delivery across both intervention arms. The absolute changes in these individual risk factors were assessed and compared pre-post between the intervention arms.
Methodology:
In Aim 2, we employed a pre-post design focusing on pre-diabetic and/or pre-hypertensive employees within the worksite. The process involved eligibility screening, baseline assessments, as well as anthropometric and laboratory evaluations. we recruited and further divided 576 eligible participants into two intervention arms: one intervention arm received canteen intervention plus behavioral change intervention (Group 1). The Group 1 included intensive education sessions, counselling, goal setting, and behaviour monitoring to facilitate change. We implemented a rigorous process tracking system throughout the intervention delivery period. The other intervention arm received canteen only intervention (Group 2), which entailed dietary modifications and the promotion of healthy eating through Information Education and Communication (IEC) methods. A thorough post-intervention assessment was conducted to evaluate the outcomes after completing both the intervention.
Outcome:
Canteen plus behaviour change intervention was implemented in Group 1, and Canteen only intervention was implemented in Group 2. Pre and Post-intervention data collection was completed.
Type of Study: Behavioural Mixed method.
Study Participants:
Pre-diabetic and/or pre-hypertensive permanent or contractual employees with a minimum work contract of two years.
Collaborators:
1. Durban University of Technology, South Africa.
2. Yale School of Public Health, USA.
Principal Investigators:
Dr. Mangesh S. Pednekar, Dr. Donna Spiegelman
Co-Investigators:
Dr. Prakash C. Gupta, Dr. Ashika Naicker
Current status: (July 2024- December 2024)
1. Data collected at different time points is under analysis.
About the study:
The main objective of the study is to examine the perceived effectiveness of the text warning on areca nut products and compare them with mandated pictorial warning labels on smokeless tobacco products.
Type of study:
Cross-sectional Study
Timeline:
Jan 01, 2022 to Dec 31, 2022
Collaborators:
Indian Council of Medical Research (ICMR)
Principal Investigators:
Dr. Namrata Puntambekar
Co- Investigators:
Dr. Prakash C. Gupta, Dr. Mangesh S. Pednekar
Study Participants:
18 years and above (only participants from Mumbai city)
Current Status:
As of September 2022
Following work has been completed in the past six months:
• Required assets have been purchased for the project
• Preparation of the script for the survey
• Preparation of the consent form
• Preparation of the training manual for field investigators
• Preparation of the sampled areas to conduct the fieldwork and drew the boundaries
• Preparation of the household enumeration form for the survey.
About the study:
Epidemiological overview:
The vaccine hesitancy towards the COVID-19 vaccination is a global problem. A systematic review in 2021 reported that only approximately 50% to 60% of all respondents worldwide would be willing to receive a COVID-19 vaccine. Most estimates suggested that reaching a 60–70% threshold of vaccinated individuals was necessary for lifestyles to return to normal, through high-coverage vaccination drives. Therefore, it is vital to understand region-specific, knowledge, attitudes, beliefs, and potential barriers that affect the uptake of COVID-19 vaccination.
Context and Problem Statement:
The national statistics have reported that among the total Indian population, 74.44 % had received their 1st dose and only 30.46% had received both doses (COWIN-dashboard;18 -10-2021). Maharashtra which is the second largest populated state in the country has remained one of the worst affected states (COVID-19 dashboard, India) right from the beginning of the pandemic with the reasons being, high population density, mobility, international arrivals/departures, mass flouting of COVID-19 norms and the seasonal flu in some regions. Although, the state as of 18th October 2021 had achieved the second-highest coverage (First Dose: 6,36,14,931 (74.82 %); Second dose: 2,84,44,097 (33.53 %) (COWIN-dashboard; 18-10-2021) of vaccination, it is inadequate to achieve herd immunity considering the large population. Therefore, understanding the hesitancy factors towards vaccination was essential to formulate effective strategies to achieve full vaccine coverage in the state and to inform the planning of vaccination drives for future pandemics.
Aim:
The study aims to understand the factors contributing to vaccine hesitancy regarding COVID-19 vaccination among the adult population of Maharashtra
Specific Study Objectives:
To study the knowledge, attitude, beliefs and factors related to COVID-19 vaccination hesitancy among adults of 18 years and above across Maharashtra.
Approach:
We used a cross-sectional online data collection approach and collected the relevant data from participants across the state of Maharashtra.
Research Question:
The study answered the research question; What are the vaccine hesitancy factors for COVID-19 vaccination among the adult population in Maharashtra?
Methodology:
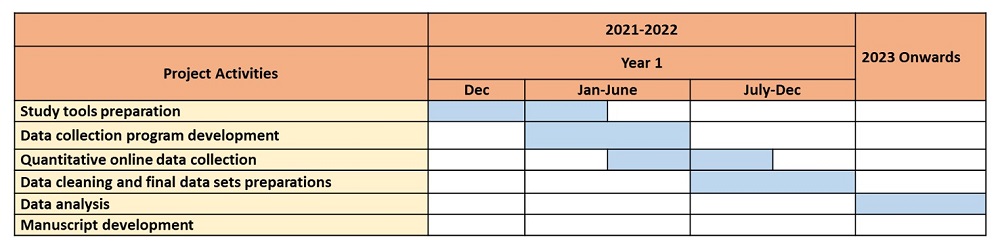
A survey tool was developed containing questions related to demographic background, perceptions, beliefs, attitudes, and factors related to vaccine hesitancy towards COVID-19 vaccination. Further, we developed an online data collection program using Redcap software. We used a snowball-probability sampling method to recruit the study sample, wherein the Healis team used WhatsApp, Facebook, LinkedIn, Instagram, the Healis website, and email to advertise and circulate the survey link to their network members. Furthermore, these network members helped in the distribution of the survey invitation to all their contacts throughout Maharashtra. The survey link remained active for 3 months (March to June 2024) for data collection. All the collected data has been securely stored on the online server at Healis and is been utilized for subsequent analyses.
Expected Outcomes:
With the study results, we were able to understand the beliefs/hesitancy factors towards the COVID-19 vaccination of the targeted study population.
Type of Study:
Epidemiological Analytic – Cross-Sectional study design
Study Participants:
Adults of 18 years and above, who agreed to voluntarily participate in the online data collection were included.
Collaborators:
The project is funded by Healis Sekhsaria Institute for Public Health (In-house)
Principal Investigators:
Dr. Khushbu Sharma
Co- Investigators:
: Dr. Mangesh. S. Pednekar, Dr. Prakash C. Gupta, Dr. Rupesh Mahajan, Mr. Sameer Narake
Current Status: (July 2024- Dec 2024)
Ongoing data analysis